| DERL2 |
|---|
|
| Identifiers |
|---|
| Aliases | DERL2, F-LAN-1, F-LANa, FLANa, CGI-101, DERtrin-2, derlin 2, derlin-2 |
|---|
| External IDs | OMIM: 610304; MGI: 2151483; HomoloGene: 9356; GeneCards: DERL2; OMA:DERL2 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 17 (human)[1] |
|---|
| | Band | 17p13.2 | Start | 5,471,254 bp[1] |
|---|
| End | 5,486,811 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 11 (mouse)[2] |
|---|
| | Band | 11 B4|11 43.21 cM | Start | 70,897,990 bp[2] |
|---|
| End | 70,910,667 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - right testis
- left testis
- body of pancreas
- Achilles tendon
- right lobe of liver
- parotid gland
- mucosa of transverse colon
- olfactory zone of nasal mucosa
- right adrenal gland
- granulocyte
|
| | Top expressed in | - cumulus cell
- left lobe of liver
- seminal vesicula
- lacrimal gland
- decidua
- right lung lobe
- stroma of bone marrow
- islet of Langerhans
- right kidney
- yolk sac
|
| | More reference expression data |
|
|---|
| BioGPS | 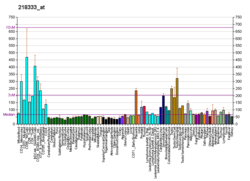 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - protein binding
- serine-type endopeptidase activity
| | Cellular component | - integral component of membrane
- integral component of endoplasmic reticulum membrane
- late endosome
- early endosome
- endoplasmic reticulum membrane
- endoplasmic reticulum
- membrane
- endoplasmic reticulum quality control compartment
- signal recognition particle receptor complex
- signal recognition particle
| | Biological process | - endoplasmic reticulum unfolded protein response
- positive regulation of cell growth
- retrograde protein transport, ER to cytosol
- response to unfolded protein
- negative regulation of retrograde protein transport, ER to cytosol
- positive regulation of cell population proliferation
- ubiquitin-dependent ERAD pathway
- suckling behavior
- endoplasmic reticulum mannose trimming
- proteolysis
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | |
|---|
NM_001304777
NM_001304779
NM_016041 |
| |
|---|
NM_001291146
NM_001291147
NM_001291148
NM_033562 |
|
|---|
| RefSeq (protein) | |
|---|
NP_001291706
NP_001291708
NP_057125 |
| |
|---|
NP_001278075
NP_001278076
NP_001278077
NP_291040 |
|
|---|
| Location (UCSC) | Chr 17: 5.47 – 5.49 Mb | Chr 11: 70.9 – 70.91 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|

















